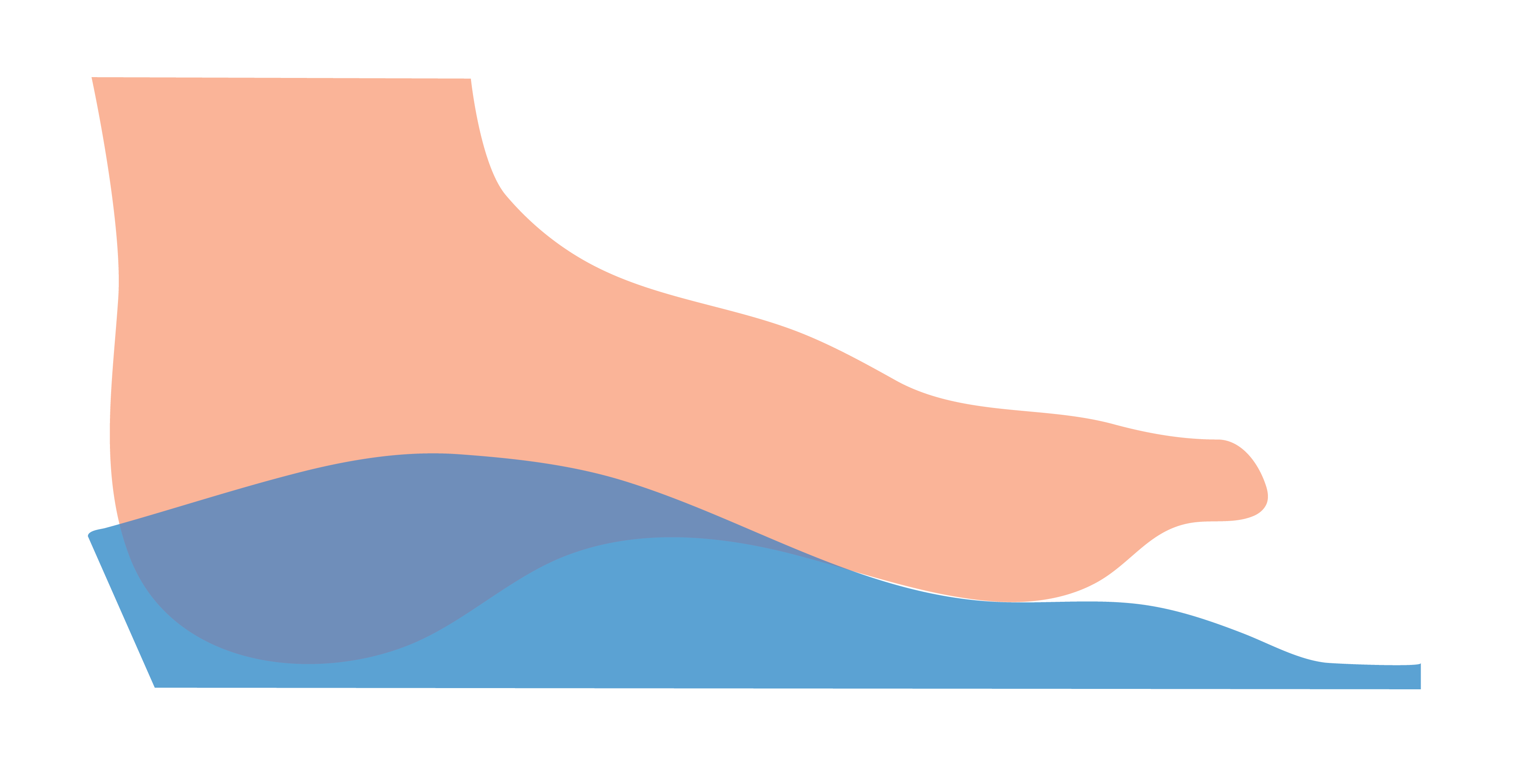
Laminated Arch
A Laminated Arch requests the adhesion of EVA to the medial longitudinal arch (MLA) to ensure that the contour of the MLA matches the 3D scan morphology.
It is good practice to request a Laminated Arch if you would like your Machined EVA orthoses to closely conform to the MLA region of the foot.
| Available Selection | Min Value | Max Value |
|---|---|---|
| None (Default) | ||
| Yes (Morphology Based) | ||
| Yes (Specific Height) | 20mm | 90mm |
None
This is the default selection and indicates that the MLA will not be laminated being the standard height of the EVA used during machining.
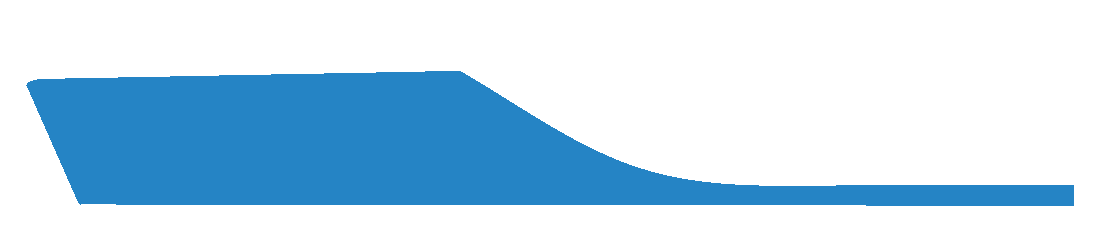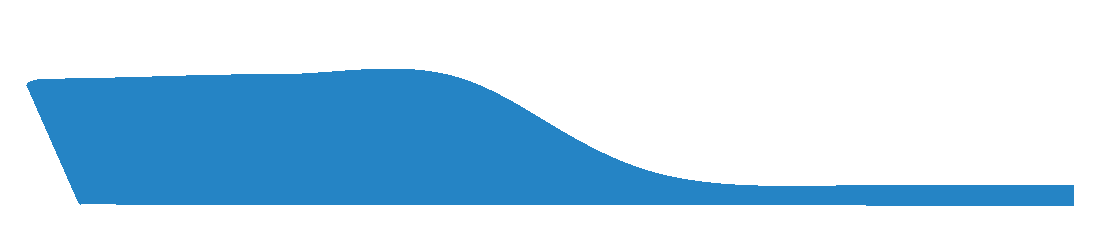
Not requesting a Laminated Arch can result in a less conformational MLA.
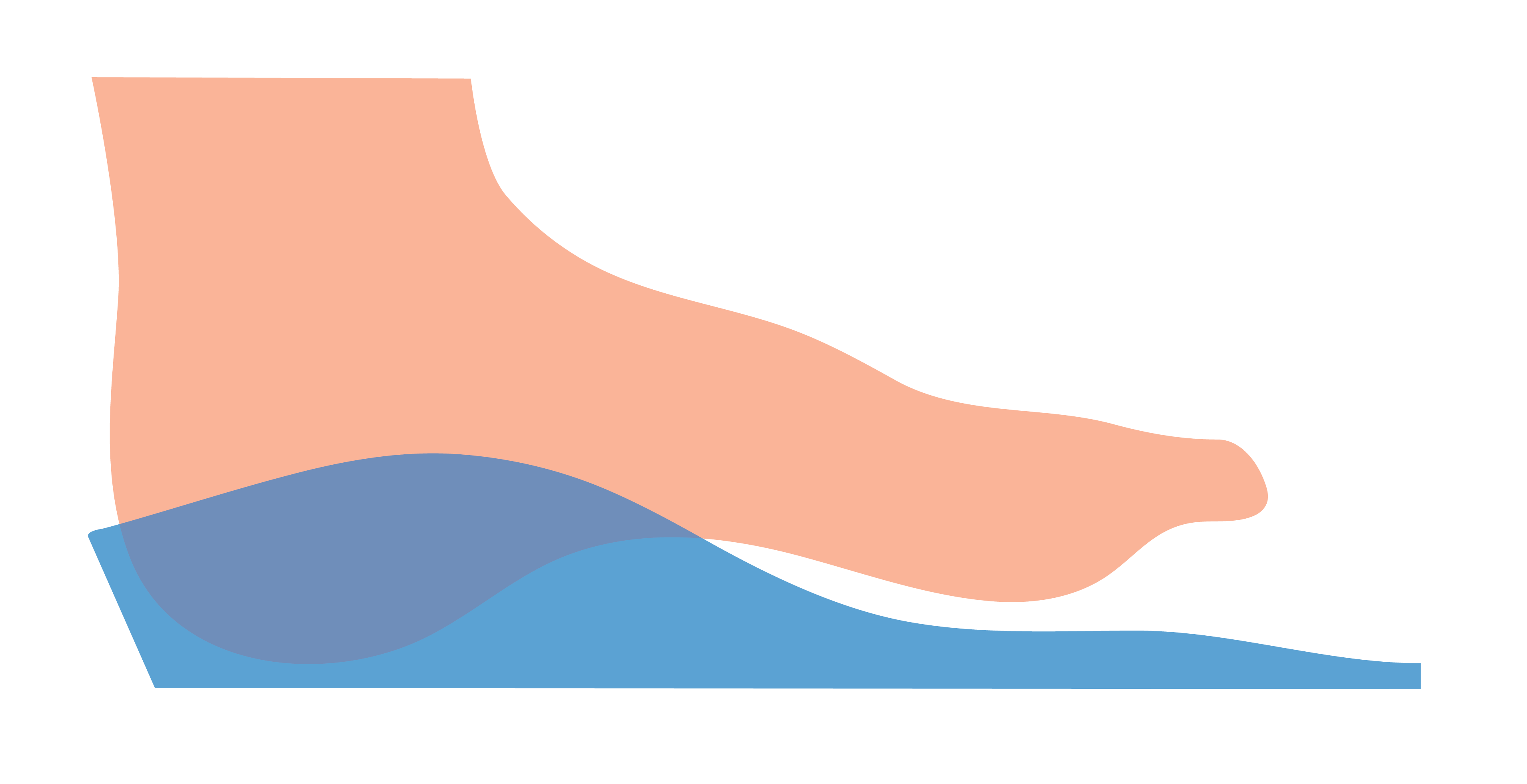
Yes (Morphology Based)
This selection indicates that a user requires the medial arch to be laminated if required, and to follow the morphology of the foot.
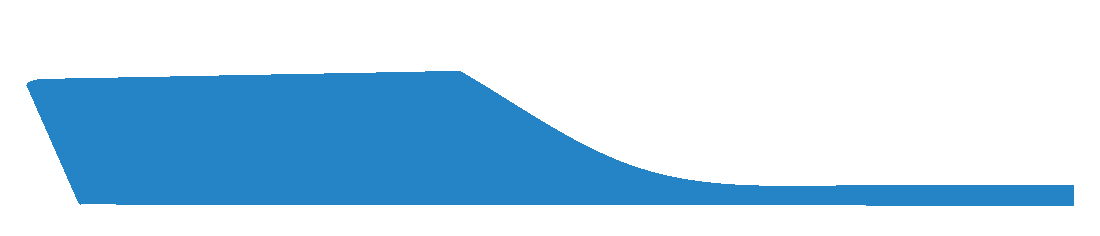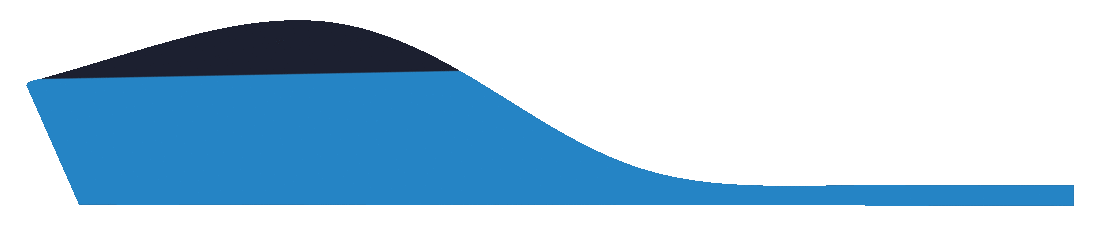
Some laboratories will provide a Laminated Arch as a default. We recommend you confirm with your laboratory as to their specific standards.
Yes (Specific Height)
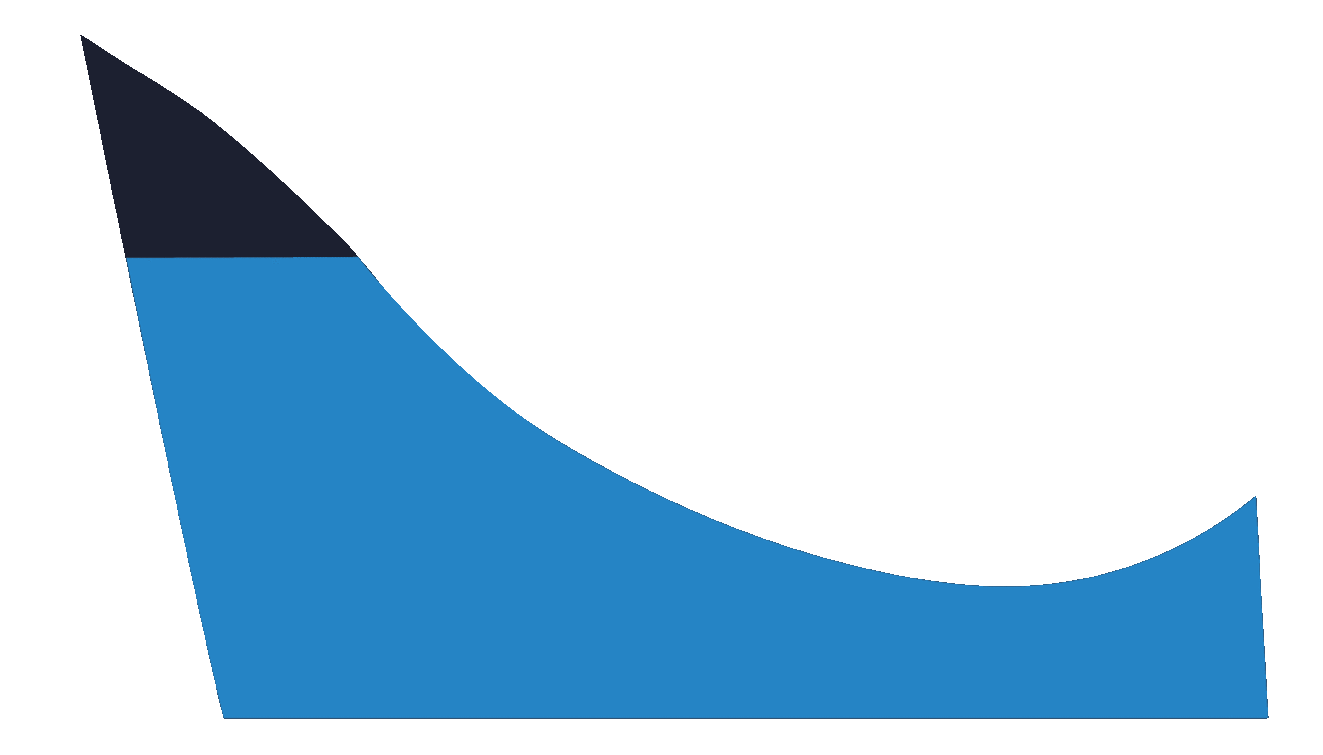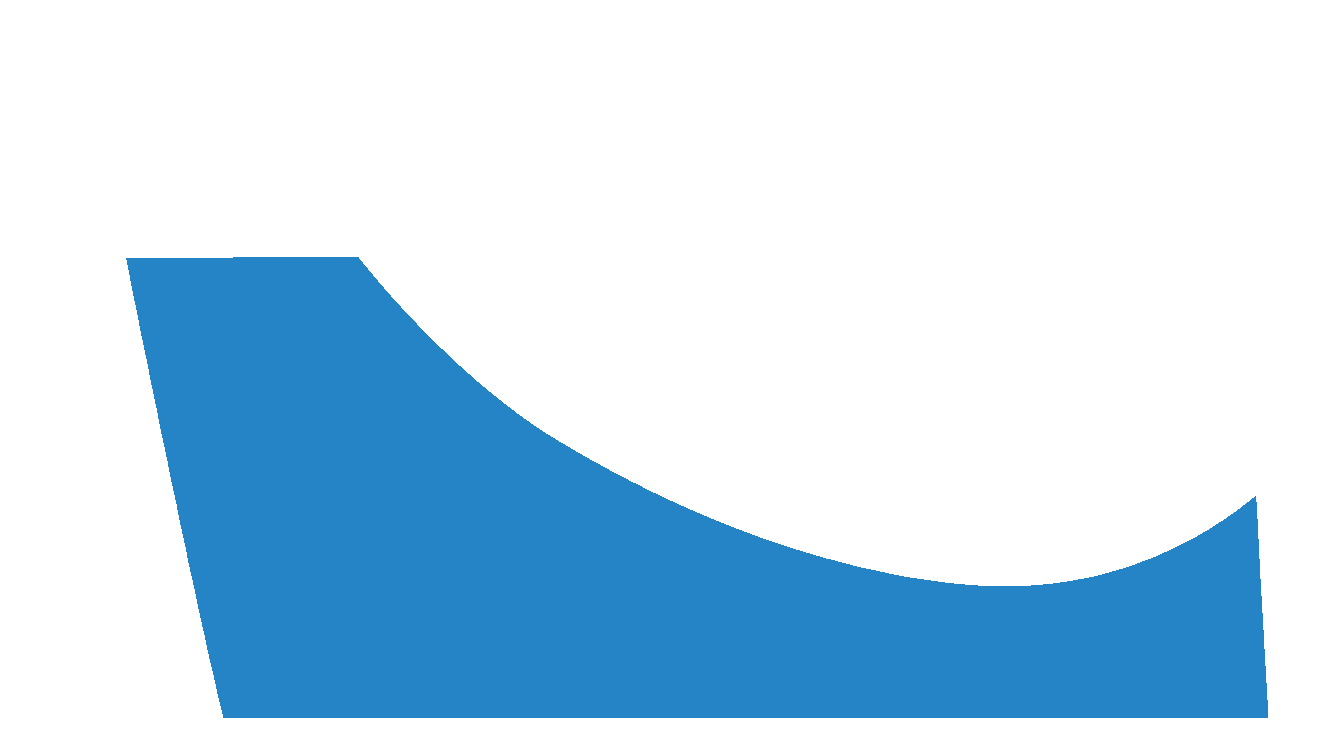
This selection indicates that a user requires the medial arch to be laminated to a specific height in millimetres.

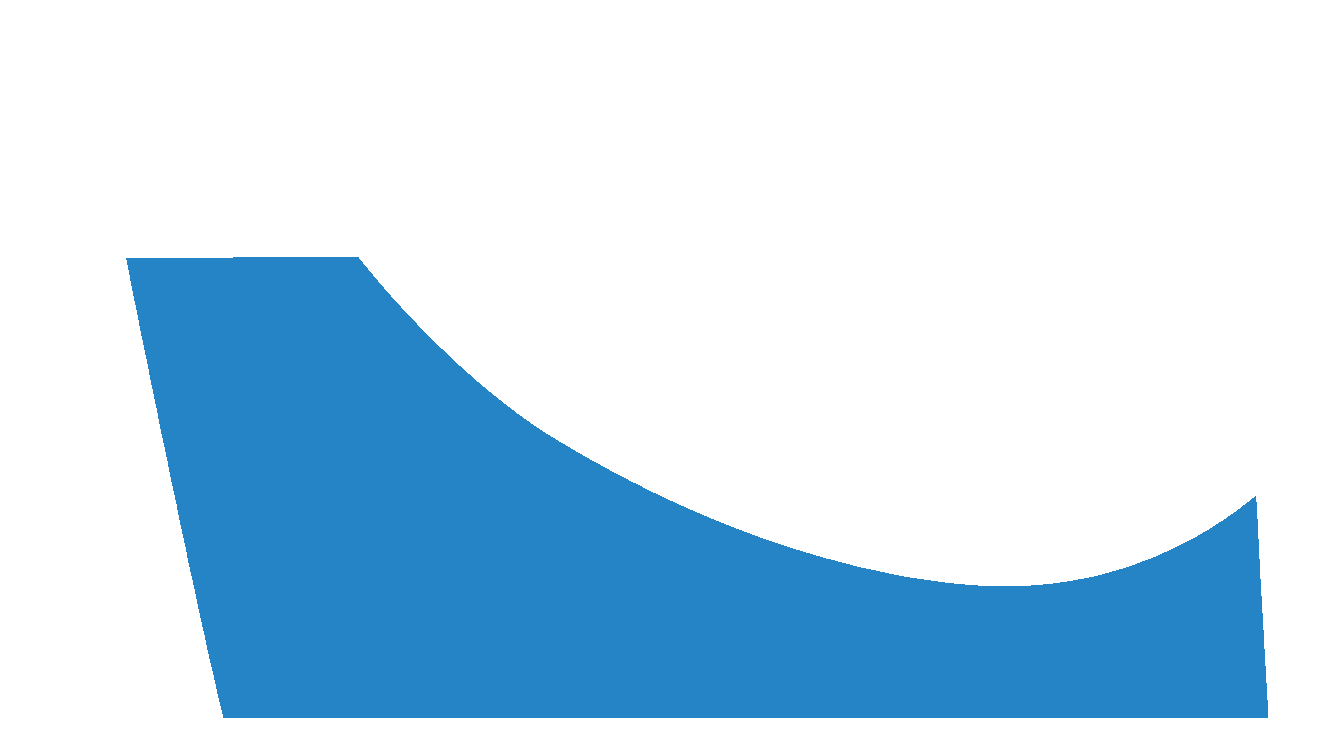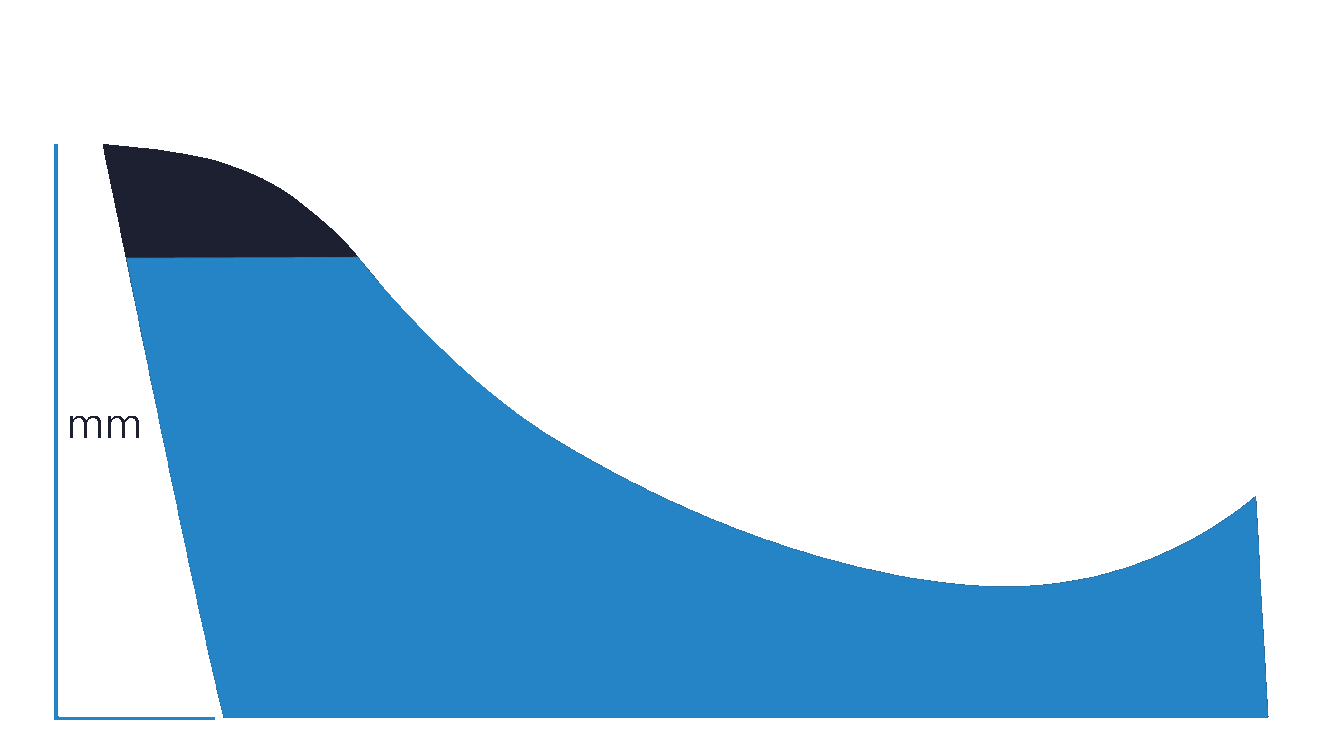
If you are unsure about what height to request, try selecting Yes (Morphology Based) instead.
First Ray Grind
A First Ray Grind requests the removal of EVA from the midpoint of the first metatarsal and is specified in millimetres.
Default Value | Minimum Value | Maximum Value |
|---|---|---|
| 0mm | 0mm | 20mm |
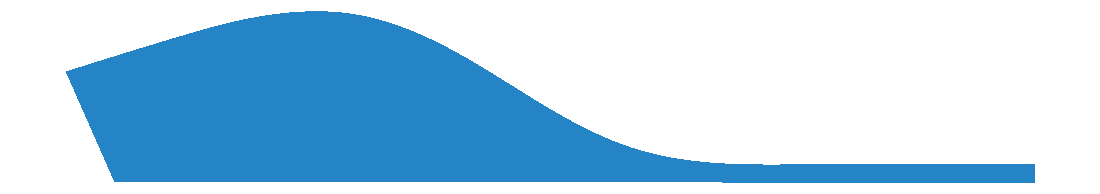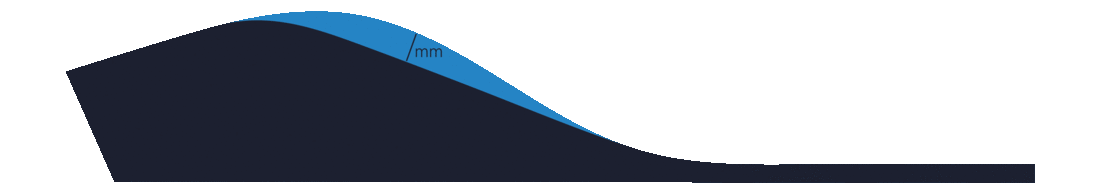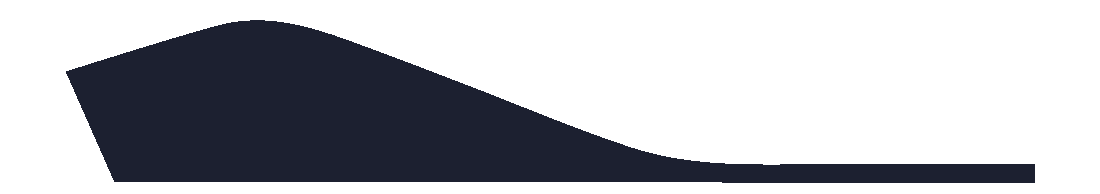
A great alternative to a First Ray Grind is to plantarflex the first metatarsal during 3D scan capture.
Maintain Plantarflexed First Ray
The Maintain Plantarflexed First Ray option can be selected when a user would like the transverse arch to be intrinsically maintained based on the foot morphology.
As a general rule, the transverse arch that will be maintained intrinsically will be more pronounced as the forefoot valgus angle increases. Plantarflexing the first metatarsal during 3D scan capture can also result in an increase in the transverse arch height relative to the 1st and 5th metatarsal heads.
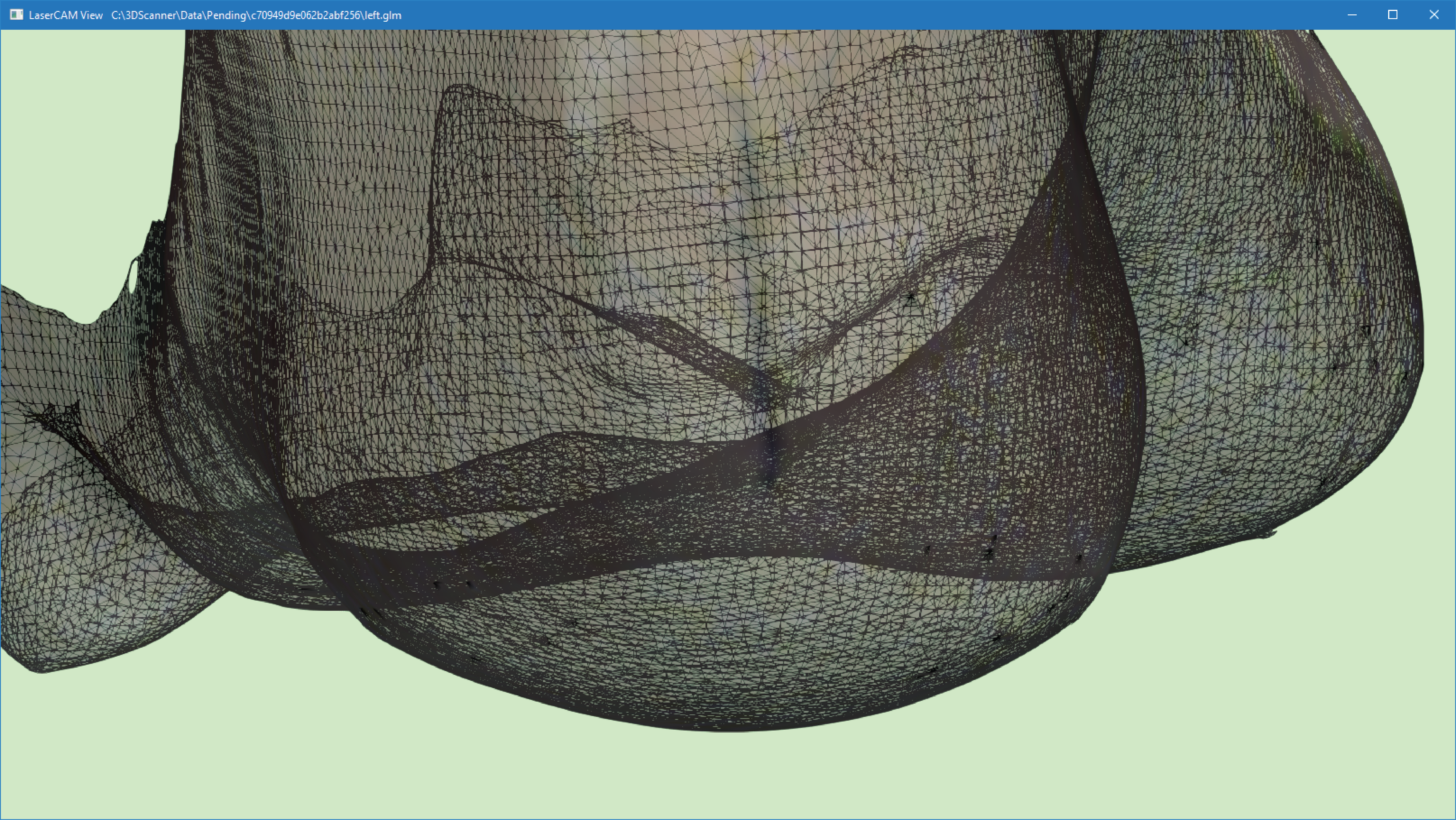
The image below shows a wireframe view of a 3D scan with a forefoot valgus alignment.
The image below shows the transverse arch region in red, which will be maintained if Maintain Plantarflexed First Ray is selected.
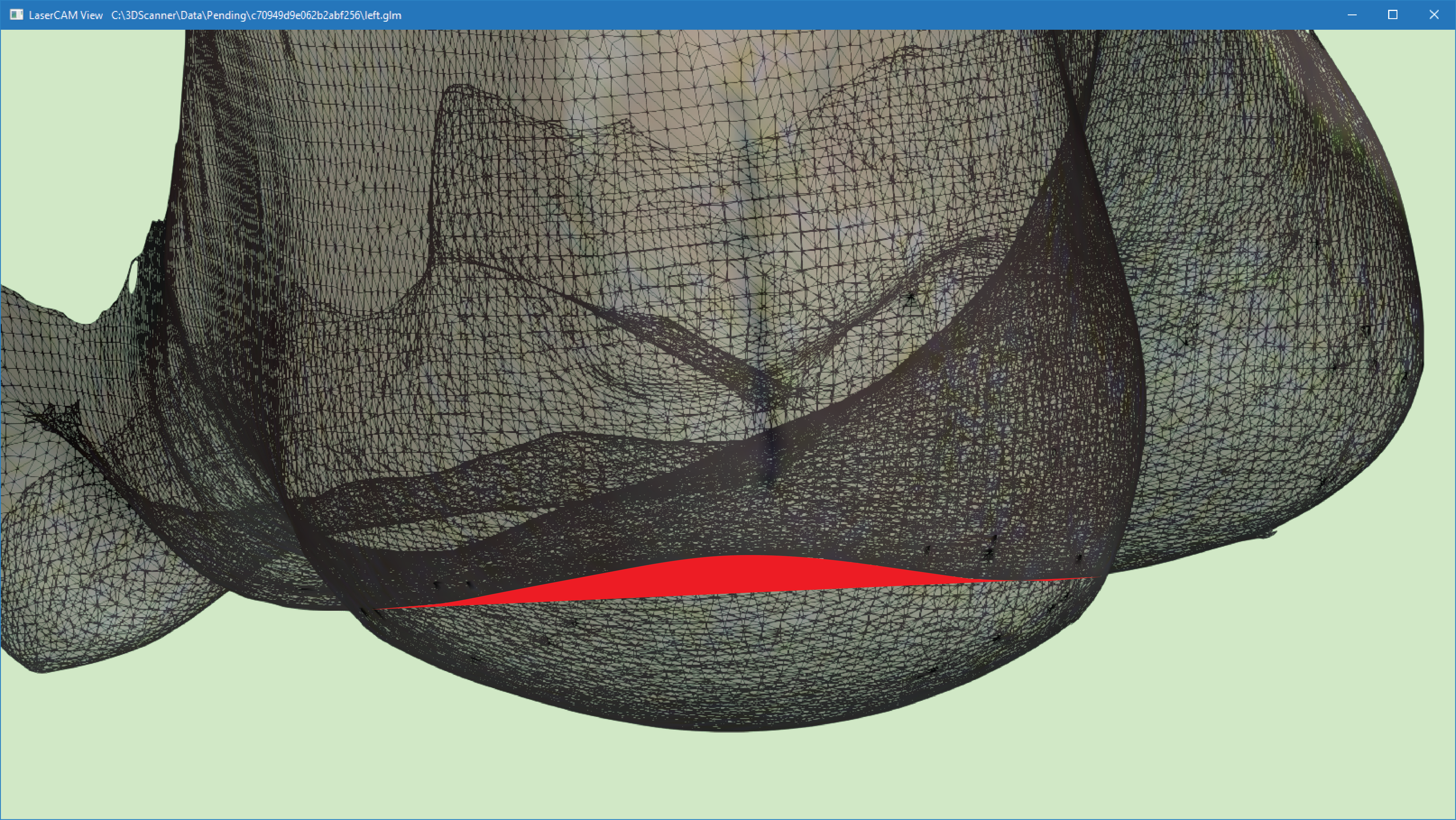
This will be positioned according to individual foot morphology, however the image below provides an approximation of the area of this transverse arch.

Maintain Forefoot Varus/Valgus
The Maintain Forefoot Varus/Valgus option allows a user to have a greater level of control regarding the specificity of any intrinsically captured Forefoot Varus or Forefoot Valgus morphology.
Available Selections |
|---|
| None (Default) |
| Morphology Based |
| Specific Thickness (mm) |
You must have captured a Forefoot Varus or Forefoot Valgus if requesting this Device Option.
None
This is the default selection and allows a user to request that no Forefoot Varus or Forefoot Valgus be maintained intrinsically within a Machined EVA orthosis.
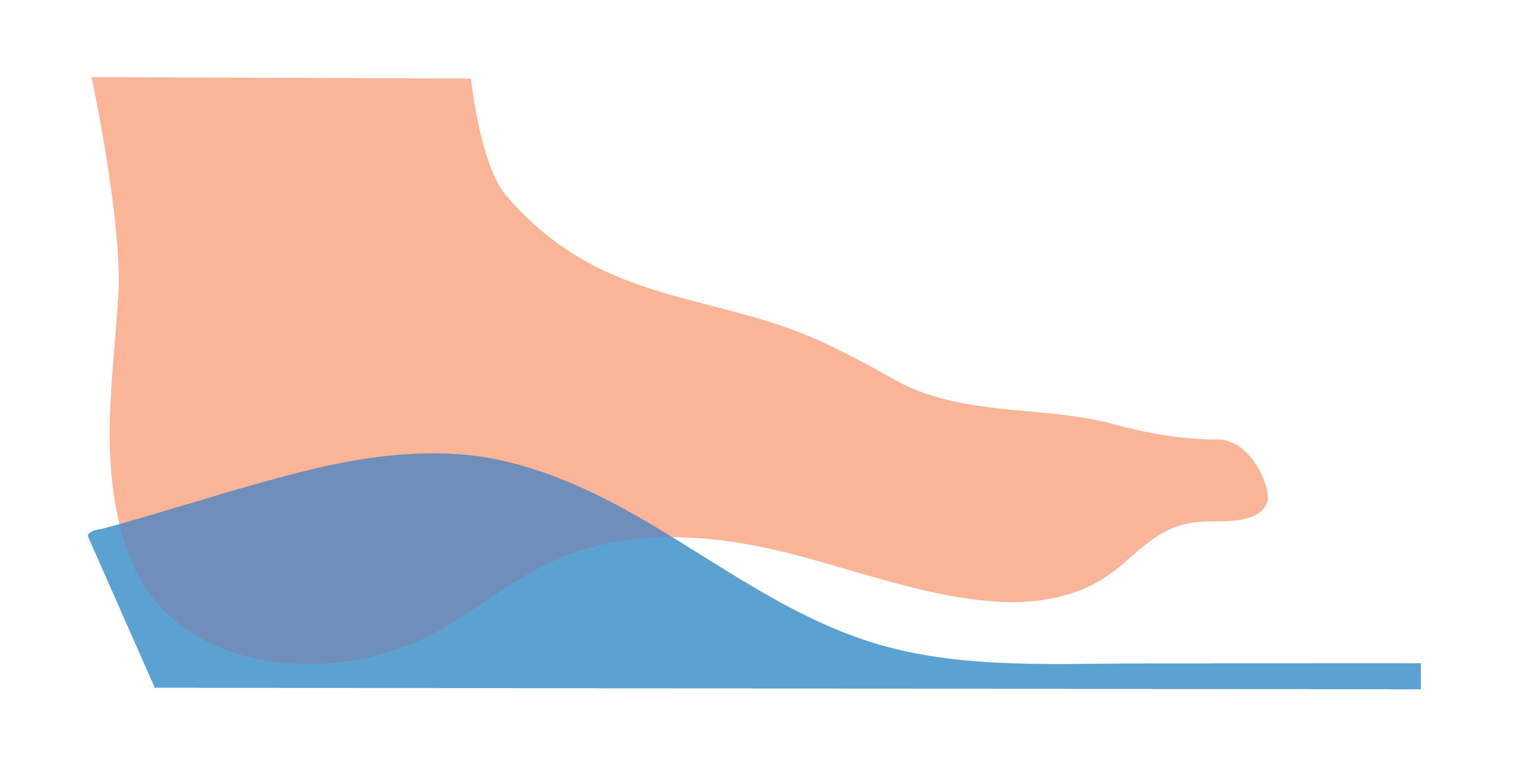
Morphology Based
This allows a user to request that any intrinsically captured Forefoot Varus or Forefoot Valgus be maintained intrinsically within a Machined EVA orthosis.
Available Selections |
|---|
| Standard Taper (Default) |
| Taper to Sulcus |
| Taper to Distal Edge |
| No Taper |
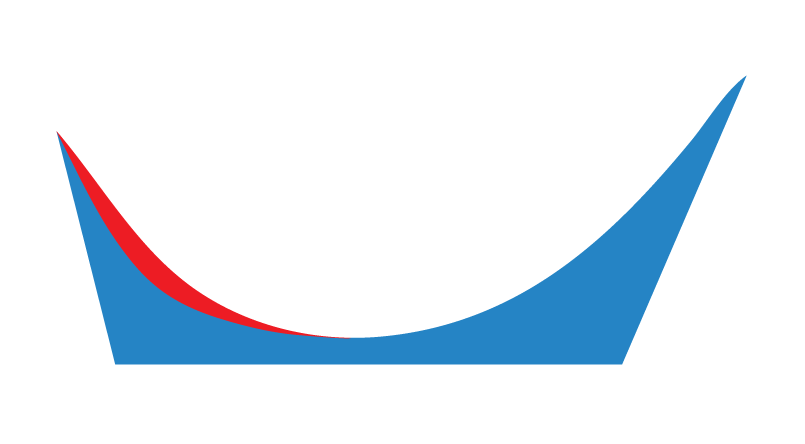
The image above represents a Forefoot Varus.
Standard Taper
This allows a user to request that a laboratory taper the intrinsically captured Forefoot Varus or Forefoot Valgus wedging as per their discretion.
Taper to Sulcus
This allows a user to request that a laboratory taper the intrinsically captured Forefoot Varus or Forefoot Valgus to the sulcus as per their discretion.
Taper to Distal Edge
This allows a user to request that a laboratory taper the intrinsically captured Forefoot Varus or Forefoot Valgus to the distal edge of the orthosis, leading to a more gradual taper.
No Taper
This allows a user to request that a laboratory taper the intrinsically captured Forefoot Varus or Forefoot Valgus suddenly from the centre of the 1st (Forefoot Varus) or 5th (Forefoot Valgus) metatarsal head.
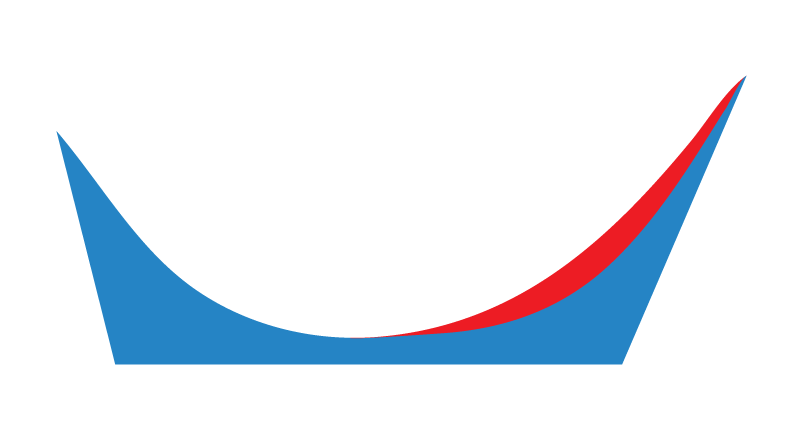
Specific Thickness (mm)
This option can only be selected if a 3D scan has a Forefoot Varus or Forefoot Valgus alignment, or the 3D scan is everted to create a Forefoot Varus or Forefoot Valgus alignment. If these conditions are not met, then an Inversion Ramp or Eversion Ramp are potentially superior prescriptive options as they allow for a more objective and less manual addition of this type of addition.
This allows a user to request that a laboratory taper the intrinsically captured Forefoot Varus or Forefoot Valgus to a specific thickness in millimetres (mm).
The image above represents a Forefoot Varus.
This is often used in combination with a First Ray Extension manufactured from PPT that comes into direct contact with the foot, and a Morton’s Extension manufactured from polypropylene on the plantar surface.
Heel cup height
Heel Cup Height
The Heel Cup Height option allows a user to select from non-specific or specific heel cup height selections.
| Available Selections | Minimum Value | Maximum Value |
|---|---|---|
| Standard (Default) | ||
| Maintain Maximum Height | ||
| Specific Thickness (mm) | 0mm | 30mm |
Standard
This allows a user to request that a laboratory lower the height of the medial and lateral heel as deemed appropriate for the strength and best application of top cover materials.
Please note that this heel cup height is dependent on the Orthosis Top Width (US) selection. An Orthosis Top Width (US) selection that is too narrow for the patients foot will result in a low heel height.
Try requesting an increase in Orthosis Top Width (US) in order to achieve a higher heel cup.
Maintain Maximum Height
This allows a user to request that a laboratory maintain the maximum height possible at the medial and lateral heel.
Specific Thickness (mm)
This allows a user to request a specific medial and lateral heel height.
If the Orthosis Top Width (US) is too narrow, then a Specific Thickness (mm) may not be achievable due to a lack of material.
Extra Heel Expansion
The Extra Heel Expansion option allows a user to request additional material be removed from the internal aspect of the medial, rear and lateral heel region of an orthosis.
Available Selections | Default Value | Minimum Value | Maximum Value |
|---|---|---|---|
| Lateral | 0mm | 0mm | 10mm |
| Medial | 0mm | 0mm | 10mm |
| Rear | 0mm | 0mm | 10mm |
Please note that you can select Lateral, Medial and Rear in a single prescription if required.
Lateral
This allows a user to request that a laboratory remove a specific thickness of material from the internal lateral heel cup.
The red region indicates the region of the heel that is manually removed when requesting this option.
This is the region of the foot that expands the most under weight-bearing conditions. It is recommended that you consider the soft tissue expansion more closely if capturing patient 3D scans or casts from a non-weight bearing position.
Medial
This allows a user to request that a laboratory remove a specific thickness of material from the internal medial heel cup.
The red region indicates the region of the heel that is manually removed when requesting this option.
Please note that requesting this option may effect the degree of Rearfoot Posting that may be intrinsically present in the same region of the orthosis.
Rear
This allows a user to request that a laboratory remove a specific thickness of material from the internal rear heel cup.
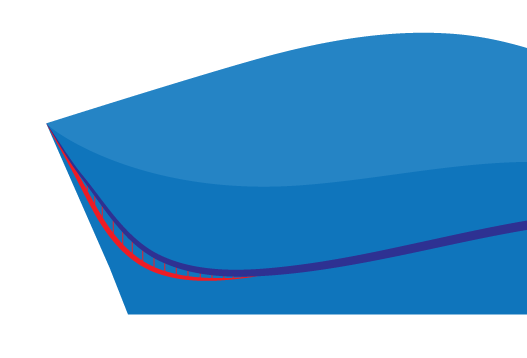
The red region indicates the region of the heel that is manually removed when requesting this option.
This is performed manually by technicians and is commonly measured using thickness callipers for accuracy.
MLA Bulk Reduction
The MLA Bulk Reduction option allows a user to request that material be removed intrinsically from either the medial, lateral or both sides of the Plantar Fascia Accommodation in the MLA.
Available Selections | Default Value | Minimum Value | Maximum Value |
|---|---|---|---|
| Medial | 0mm | 0mm | 10mm |
| Lateral | 0mm | 0mm | 10mm |
| Both | 0mm | 0mm | 10mm |
None
No material will be removed from the MLA on either side of the Plantar Fascia Accommodation.
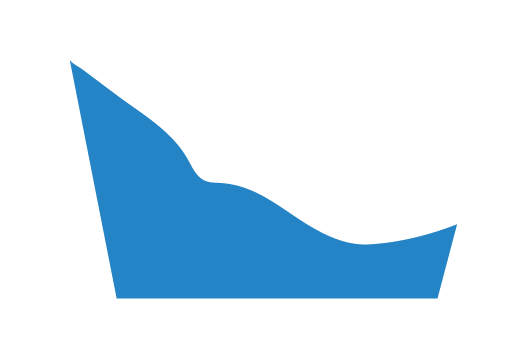
The image above represents a cross section of the foot orthosis in the frontal plane. The medial side is on the left and the lateral side is on the right.
Medial
A specified thickness of material will be removed from the MLA on the medial side of the Plantar Fascia Accommodation.
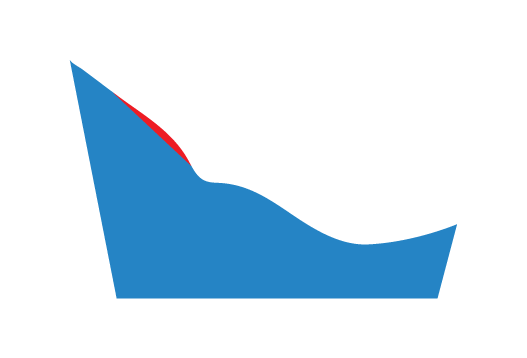
The red region represents the material removed by selecting this option.
Lateral
A specified thickness of material will be removed from the MLA on the lateral side of the Plantar Fascia Accommodation.
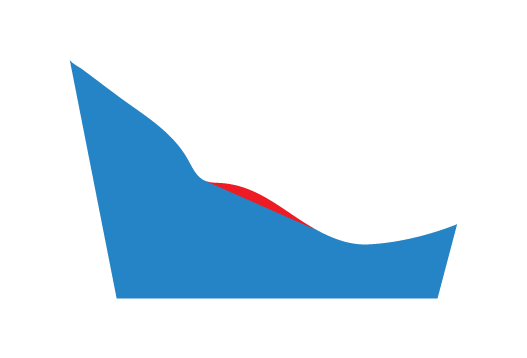
The red region represents the material removed by selecting this option.
Both
A specified thickness of material will be removed from the MLA on the both sides of the Plantar Fascia Accommodation.
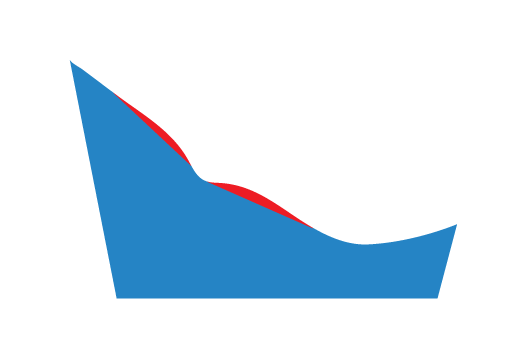
The red region represents the material removed by selecting this option.
